Pharmacovigilance Market Overview:
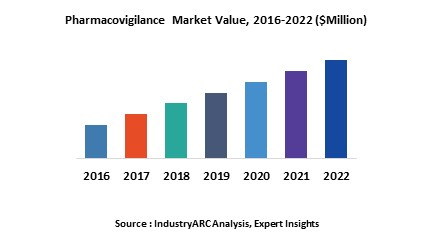
According to the U.S.-based Public Citizen’s Health Research Group, approximately two million people are affected by adverse drug reactions (ADR), which includes 100,000 fatalities, in the U.S. every year.[1] Traditionally, healthcare companies have utilized in-house and outsourcing pharmacovigilance services for effectively monitoring the positive effects as well as the side-effects of licensed medical drugs. Pharmacovigilance is a type of clinical research which determines drug safety and associated medical hazards, plans risk management, and anticipates remodification of drugs. It is aiding healthcare providers in easy detection and targeted spontaneous reporting of medication errors, thereby reducing risk of fatality. While the global pharmacovigilance market stood at $4.3 billion in 2018, the increasing scope of opportunities estimate the market to grow at a CAGR of 8.8% through to 2025.
ADR reporting is gaining increased end user appeal due to strict government policies for drug safety approval and testing. Approximately 61,311 people died due to drug poisoning in the United States as per the Insurance Information Institute.[2] North America, with a share of 37%, is the leading contributor to the pharmacovigilance market, followed by Europe and the Asia-Pacific region, in 2018. The outsourcing pharmacovigilance segment will generate sustainable demand, which is estimated to help the market grow at a CAGR of 8.8% during the forecast period 2019 to 2025.
Pharmacovigilance Market Growth Drivers:
· Stringent government regulations regarding drug administration, and increasing incidences of adversities resulting from from medication is boosting pharmacovigilance services, thereby providing greater possibilities for expanding the market size.
· Increasing medical errors, developing healthcare infrastructure and increasing pharmaceutical production are reducing fatal ADR incidents, and causing a positive impact on the pharmacovigilance market.
Pharmacovigilance Market Key Players:
Leading companies in the pharmacovigilance market include Bristol- Myers Squibb, Boehringer Ingelheim, TCS, Cognizant, United BioSource, Covance, IQVIA (Quintiles IMS), Accenture,Paraxel, Wipro Limited, and others.
Bristol- Myers Squibb is a leading pharmaceutical manufacturing company which provides various products and solutions to end users, alongside conducting independent research on products and therapeutic methods.
Pharmacovigilance Market Trends:
· AI Enabled Pharmacovigilance:
Innovation in automation of mechanical procedures has led to advent of Artificial Intelligence (AI) methods, which are all set to take over the healthcare pharmacovigilance operative service. Genpact, a global digital transformation platform for industries, delivers Cora Pharmacovigilance facility for achieving quality insights and assessing, configuring, and signaling adverse events, thereby prioritizing patient safety. With its ability to provide end to end pharmacovigilance services, this technology can be incorporated with leading systems through AI and machine learning operations.
Pharmacovigilance Market Research Scope:
The base year of the study is 2018, with forecast done up to 2025. The study presents a thorough analysis of the competitive landscape, taking into account the market shares of the leading companies. It also provides information on unit shipments. These provide the key market participants with the necessary business intelligence and help them understand the future of the Pharmacovigilance Market. The assessment includes the forecast, an overview of the competitive structure, the market shares of the competitors, as well as the market trends, market demands, market drivers, market challenges, and product analysis. The market drivers and restraints have been assessed to fathom their impact over the forecast period. This report further identifies the key opportunities for growth while also detailing the key challenges and possible threats. The key areas of focus include the types of pharmacovigilance services in Pharmacovigilance Market, and their specific applications in different areas.
Pharmacovigilance Market: Industry Coverage:
Global pharmacovigilance market is basically classified into clinical trial phases, and types of service providers. On the basis of clinical trial phases, categorization includes preclinical studies, phase I/1, phase II/2, phase III/3, and phase IV/4 trial. Based on the type of service providers bifurcation includes in-house, contract outsourcing, and others.
The Pharmacovigilance Market also analyzes the major geographic regions for the market as well as the major countries for the market in these regions. The regions and countries covered in the study include:
• North America: The U.S., Canada, Mexico
• South America: Brazil, Venezuela, Argentina, Ecuador, Peru, Colombia, Costa Rica
• Europe: The U.K., Germany, Italy, France, The Netherlands, Belgium, Spain, Denmark
• APAC: China, Japan, Australia, South Korea, India, Taiwan, Malaysia, Hong Kong
• Middle East and Africa: Israel, South Africa, Saudi Arabia
[1] http://www.worstpills.org
[2] https://www.iii.org
Table 1 Pharmacovigilance Market Overview 2023-2030
Table 2 Pharmacovigilance Market Leader Analysis 2023-2030 (US$)
Table 3 Pharmacovigilance Market Product Analysis 2023-2030 (US$)
Table 4 Pharmacovigilance Market End User Analysis 2023-2030 (US$)
Table 5 Pharmacovigilance Market Patent Analysis 2013-2023* (US$)
Table 6 Pharmacovigilance Market Financial Analysis 2023-2030 (US$)
Table 7 Pharmacovigilance Market Driver Analysis 2023-2030 (US$)
Table 8 Pharmacovigilance Market Challenges Analysis 2023-2030 (US$)
Table 9 Pharmacovigilance Market Constraint Analysis 2023-2030 (US$)
Table 10 Pharmacovigilance Market Supplier Bargaining Power Analysis 2023-2030 (US$)
Table 11 Pharmacovigilance Market Buyer Bargaining Power Analysis 2023-2030 (US$)
Table 12 Pharmacovigilance Market Threat of Substitutes Analysis 2023-2030 (US$)
Table 13 Pharmacovigilance Market Threat of New Entrants Analysis 2023-2030 (US$)
Table 14 Pharmacovigilance Market Degree of Competition Analysis 2023-2030 (US$)
Table 15 Pharmacovigilance Market Value Chain Analysis 2023-2030 (US$)
Table 16 Pharmacovigilance Market Pricing Analysis 2023-2030 (US$)
Table 17 Pharmacovigilance Market Opportunities Analysis 2023-2030 (US$)
Table 18 Pharmacovigilance Market Product Life Cycle Analysis 2023-2030 (US$)
Table 19 Pharmacovigilance Market Supplier Analysis 2023-2030 (US$)
Table 20 Pharmacovigilance Market Distributor Analysis 2023-2030 (US$)
Table 21 Pharmacovigilance Market Trend Analysis 2023-2030 (US$)
Table 22 Pharmacovigilance Market Size 2023 (US$)
Table 23 Pharmacovigilance Market Forecast Analysis 2023-2030 (US$)
Table 24 Pharmacovigilance Market Sales Forecast Analysis 2023-2030 (Units)
Table 25 Pharmacovigilance Market, Revenue & Volume, By Type of Service Provider, 2023-2030 ($)
Table 26 Pharmacovigilance Market By Type of Service Provider, Revenue & Volume, By In House, 2023-2030 ($)
Table 27 Pharmacovigilance Market By Type of Service Provider, Revenue & Volume, By Contract Outsourcing, 2023-2030 ($)
Table 28 Pharmacovigilance Market, Revenue & Volume, By Clinical Trial Phase, 2023-2030 ($)
Table 29 Pharmacovigilance Market By Clinical Trial Phase, Revenue & Volume, By Preclinical Studies, 2023-2030 ($)
Table 30 Pharmacovigilance Market By Clinical Trial Phase, Revenue & Volume, By Phase I/1, 2023-2030 ($)
Table 31 Pharmacovigilance Market By Clinical Trial Phase, Revenue & Volume, By Phase II/2, 2023-2030 ($)
Table 32 Pharmacovigilance Market By Clinical Trial Phase, Revenue & Volume, By Phase III/3, 2023-2030 ($)
Table 33 Pharmacovigilance Market By Clinical Trial Phase, Revenue & Volume, By Phase IV/4 Trial, 2023-2030 ($)
Table 34 North America Pharmacovigilance Market, Revenue & Volume, By Type of Service Provider, 2023-2030 ($)
Table 35 North America Pharmacovigilance Market, Revenue & Volume, By Clinical Trial Phase, 2023-2030 ($)
Table 36 South america Pharmacovigilance Market, Revenue & Volume, By Type of Service Provider, 2023-2030 ($)
Table 37 South america Pharmacovigilance Market, Revenue & Volume, By Clinical Trial Phase, 2023-2030 ($)
Table 38 Europe Pharmacovigilance Market, Revenue & Volume, By Type of Service Provider, 2023-2030 ($)
Table 39 Europe Pharmacovigilance Market, Revenue & Volume, By Clinical Trial Phase, 2023-2030 ($)
Table 40 APAC Pharmacovigilance Market, Revenue & Volume, By Type of Service Provider, 2023-2030 ($)
Table 41 APAC Pharmacovigilance Market, Revenue & Volume, By Clinical Trial Phase, 2023-2030 ($)
Table 42 Middle East & Africa Pharmacovigilance Market, Revenue & Volume, By Type of Service Provider, 2023-2030 ($)
Table 43 Middle East & Africa Pharmacovigilance Market, Revenue & Volume, By Clinical Trial Phase, 2023-2030 ($)
Table 44 Russia Pharmacovigilance Market, Revenue & Volume, By Type of Service Provider, 2023-2030 ($)
Table 45 Russia Pharmacovigilance Market, Revenue & Volume, By Clinical Trial Phase, 2023-2030 ($)
Table 46 Israel Pharmacovigilance Market, Revenue & Volume, By Type of Service Provider, 2023-2030 ($)
Table 47 Israel Pharmacovigilance Market, Revenue & Volume, By Clinical Trial Phase, 2023-2030 ($)
Table 48 Top Companies 2023 (US$)Pharmacovigilance Market, Revenue & Volume
Table 49 Product Launch 2023-2030Pharmacovigilance Market, Revenue & Volume
Table 50 Mergers & Acquistions 2023-2030Pharmacovigilance Market, Revenue & Volume
List of Figures
Figure 1 Overview of Pharmacovigilance Market 2023-2030
Figure 2 Market Share Analysis for Pharmacovigilance Market 2023 (US$)
Figure 3 Product Comparison in Pharmacovigilance Market 2023-2030 (US$)
Figure 4 End User Profile for Pharmacovigilance Market 2023-2030 (US$)
Figure 5 Patent Application and Grant in Pharmacovigilance Market 2013-2023* (US$)
Figure 6 Top 5 Companies Financial Analysis in Pharmacovigilance Market 2023-2030 (US$)
Figure 7 Market Entry Strategy in Pharmacovigilance Market 2023-2030
Figure 8 Ecosystem Analysis in Pharmacovigilance Market 2023
Figure 9 Average Selling Price in Pharmacovigilance Market 2023-2030
Figure 10 Top Opportunites in Pharmacovigilance Market 2023-2030
Figure 11 Market Life Cycle Analysis in Pharmacovigilance Market
Figure 12 GlobalBy Type of Service ProviderPharmacovigilance Market Revenue, 2023-2030 ($)
Figure 13 GlobalBy Clinical Trial PhasePharmacovigilance Market Revenue, 2023-2030 ($)
Figure 14 Global Pharmacovigilance Market - By Geography
Figure 15 Global Pharmacovigilance Market Value & Volume, By Geography, 2023-2030 ($)
Figure 16 Global Pharmacovigilance Market CAGR, By Geography, 2023-2030 (%)
Figure 17 North America Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 18 US Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 19 US GDP and Population, 2023-2030 ($)
Figure 20 US GDP – Composition of 2023, By Sector of Origin
Figure 21 US Export and Import Value & Volume, 2023-2030 ($)
Figure 22 Canada Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 23 Canada GDP and Population, 2023-2030 ($)
Figure 24 Canada GDP – Composition of 2023, By Sector of Origin
Figure 25 Canada Export and Import Value & Volume, 2023-2030 ($)
Figure 26 Mexico Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 27 Mexico GDP and Population, 2023-2030 ($)
Figure 28 Mexico GDP – Composition of 2023, By Sector of Origin
Figure 29 Mexico Export and Import Value & Volume, 2023-2030 ($)
Figure 30 South America Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 31 Brazil Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 32 Brazil GDP and Population, 2023-2030 ($)
Figure 33 Brazil GDP – Composition of 2023, By Sector of Origin
Figure 34 Brazil Export and Import Value & Volume, 2023-2030 ($)
Figure 35 Venezuela Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 36 Venezuela GDP and Population, 2023-2030 ($)
Figure 37 Venezuela GDP – Composition of 2023, By Sector of Origin
Figure 38 Venezuela Export and Import Value & Volume, 2023-2030 ($)
Figure 39 Argentina Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 40 Argentina GDP and Population, 2023-2030 ($)
Figure 41 Argentina GDP – Composition of 2023, By Sector of Origin
Figure 42 Argentina Export and Import Value & Volume, 2023-2030 ($)
Figure 43 Ecuador Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 44 Ecuador GDP and Population, 2023-2030 ($)
Figure 45 Ecuador GDP – Composition of 2023, By Sector of Origin
Figure 46 Ecuador Export and Import Value & Volume, 2023-2030 ($)
Figure 47 Peru Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 48 Peru GDP and Population, 2023-2030 ($)
Figure 49 Peru GDP – Composition of 2023, By Sector of Origin
Figure 50 Peru Export and Import Value & Volume, 2023-2030 ($)
Figure 51 Colombia Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 52 Colombia GDP and Population, 2023-2030 ($)
Figure 53 Colombia GDP – Composition of 2023, By Sector of Origin
Figure 54 Colombia Export and Import Value & Volume, 2023-2030 ($)
Figure 55 Costa Rica Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 56 Costa Rica GDP and Population, 2023-2030 ($)
Figure 57 Costa Rica GDP – Composition of 2023, By Sector of Origin
Figure 58 Costa Rica Export and Import Value & Volume, 2023-2030 ($)
Figure 59 Europe Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 60 U.K Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 61 U.K GDP and Population, 2023-2030 ($)
Figure 62 U.K GDP – Composition of 2023, By Sector of Origin
Figure 63 U.K Export and Import Value & Volume, 2023-2030 ($)
Figure 64 Germany Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 65 Germany GDP and Population, 2023-2030 ($)
Figure 66 Germany GDP – Composition of 2023, By Sector of Origin
Figure 67 Germany Export and Import Value & Volume, 2023-2030 ($)
Figure 68 Italy Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 69 Italy GDP and Population, 2023-2030 ($)
Figure 70 Italy GDP – Composition of 2023, By Sector of Origin
Figure 71 Italy Export and Import Value & Volume, 2023-2030 ($)
Figure 72 France Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 73 France GDP and Population, 2023-2030 ($)
Figure 74 France GDP – Composition of 2023, By Sector of Origin
Figure 75 France Export and Import Value & Volume, 2023-2030 ($)
Figure 76 Netherlands Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 77 Netherlands GDP and Population, 2023-2030 ($)
Figure 78 Netherlands GDP – Composition of 2023, By Sector of Origin
Figure 79 Netherlands Export and Import Value & Volume, 2023-2030 ($)
Figure 80 Belgium Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 81 Belgium GDP and Population, 2023-2030 ($)
Figure 82 Belgium GDP – Composition of 2023, By Sector of Origin
Figure 83 Belgium Export and Import Value & Volume, 2023-2030 ($)
Figure 84 Spain Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 85 Spain GDP and Population, 2023-2030 ($)
Figure 86 Spain GDP – Composition of 2023, By Sector of Origin
Figure 87 Spain Export and Import Value & Volume, 2023-2030 ($)
Figure 88 Denmark Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 89 Denmark GDP and Population, 2023-2030 ($)
Figure 90 Denmark GDP – Composition of 2023, By Sector of Origin
Figure 91 Denmark Export and Import Value & Volume, 2023-2030 ($)
Figure 92 APAC Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 93 China Pharmacovigilance Market Value & Volume, 2023-2030
Figure 94 China GDP and Population, 2023-2030 ($)
Figure 95 China GDP – Composition of 2023, By Sector of Origin
Figure 96 China Export and Import Value & Volume, 2023-2030 ($)Pharmacovigilance Market China Export and Import Value & Volume, 2023-2030 ($)
Figure 97 Australia Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 98 Australia GDP and Population, 2023-2030 ($)
Figure 99 Australia GDP – Composition of 2023, By Sector of Origin
Figure 100 Australia Export and Import Value & Volume, 2023-2030 ($)
Figure 101 South Korea Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 102 South Korea GDP and Population, 2023-2030 ($)
Figure 103 South Korea GDP – Composition of 2023, By Sector of Origin
Figure 104 South Korea Export and Import Value & Volume, 2023-2030 ($)
Figure 105 India Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 106 India GDP and Population, 2023-2030 ($)
Figure 107 India GDP – Composition of 2023, By Sector of Origin
Figure 108 India Export and Import Value & Volume, 2023-2030 ($)
Figure 109 Taiwan Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 110 Taiwan GDP and Population, 2023-2030 ($)
Figure 111 Taiwan GDP – Composition of 2023, By Sector of Origin
Figure 112 Taiwan Export and Import Value & Volume, 2023-2030 ($)
Figure 113 Malaysia Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 114 Malaysia GDP and Population, 2023-2030 ($)
Figure 115 Malaysia GDP – Composition of 2023, By Sector of Origin
Figure 116 Malaysia Export and Import Value & Volume, 2023-2030 ($)
Figure 117 Hong Kong Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 118 Hong Kong GDP and Population, 2023-2030 ($)
Figure 119 Hong Kong GDP – Composition of 2023, By Sector of Origin
Figure 120 Hong Kong Export and Import Value & Volume, 2023-2030 ($)
Figure 121 Middle East & Africa Pharmacovigilance Market Middle East & Africa 3D Printing Market Value & Volume, 2023-2030 ($)
Figure 122 Russia Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 123 Russia GDP and Population, 2023-2030 ($)
Figure 124 Russia GDP – Composition of 2023, By Sector of Origin
Figure 125 Russia Export and Import Value & Volume, 2023-2030 ($)
Figure 126 Israel Pharmacovigilance Market Value & Volume, 2023-2030 ($)
Figure 127 Israel GDP and Population, 2023-2030 ($)
Figure 128 Israel GDP – Composition of 2023, By Sector of Origin
Figure 129 Israel Export and Import Value & Volume, 2023-2030 ($)
Figure 130 Entropy Share, By Strategies, 2023-2030* (%)Pharmacovigilance Market
Figure 131 Developments, 2023-2030*Pharmacovigilance Market
Figure 132 Company 1 Pharmacovigilance Market Net Revenue, By Years, 2023-2030* ($)
Figure 133 Company 1 Pharmacovigilance Market Net Revenue Share, By Business segments, 2023 (%)
Figure 134 Company 1 Pharmacovigilance Market Net Sales Share, By Geography, 2023 (%)
Figure 135 Company 2 Pharmacovigilance Market Net Revenue, By Years, 2023-2030* ($)
Figure 136 Company 2 Pharmacovigilance Market Net Revenue Share, By Business segments, 2023 (%)
Figure 137 Company 2 Pharmacovigilance Market Net Sales Share, By Geography, 2023 (%)
Figure 138 Company 3 Pharmacovigilance Market Net Revenue, By Years, 2023-2030* ($)
Figure 139 Company 3 Pharmacovigilance Market Net Revenue Share, By Business segments, 2023 (%)
Figure 140 Company 3 Pharmacovigilance Market Net Sales Share, By Geography, 2023 (%)
Figure 141 Company 4 Pharmacovigilance Market Net Revenue, By Years, 2023-2030* ($)
Figure 142 Company 4 Pharmacovigilance Market Net Revenue Share, By Business segments, 2023 (%)
Figure 143 Company 4 Pharmacovigilance Market Net Sales Share, By Geography, 2023 (%)
Figure 144 Company 5 Pharmacovigilance Market Net Revenue, By Years, 2023-2030* ($)
Figure 145 Company 5 Pharmacovigilance Market Net Revenue Share, By Business segments, 2023 (%)
Figure 146 Company 5 Pharmacovigilance Market Net Sales Share, By Geography, 2023 (%)
Figure 147 Company 6 Pharmacovigilance Market Net Revenue, By Years, 2023-2030* ($)
Figure 148 Company 6 Pharmacovigilance Market Net Revenue Share, By Business segments, 2023 (%)
Figure 149 Company 6 Pharmacovigilance Market Net Sales Share, By Geography, 2023 (%)
Figure 150 Company 7 Pharmacovigilance Market Net Revenue, By Years, 2023-2030* ($)
Figure 151 Company 7 Pharmacovigilance Market Net Revenue Share, By Business segments, 2023 (%)
Figure 152 Company 7 Pharmacovigilance Market Net Sales Share, By Geography, 2023 (%)
Figure 153 Company 8 Pharmacovigilance Market Net Revenue, By Years, 2023-2030* ($)
Figure 154 Company 8 Pharmacovigilance Market Net Revenue Share, By Business segments, 2023 (%)
Figure 155 Company 8 Pharmacovigilance Market Net Sales Share, By Geography, 2023 (%)
Figure 156 Company 9 Pharmacovigilance Market Net Revenue, By Years, 2023-2030* ($)
Figure 157 Company 9 Pharmacovigilance Market Net Revenue Share, By Business segments, 2023 (%)
Figure 158 Company 9 Pharmacovigilance Market Net Sales Share, By Geography, 2023 (%)
Figure 159 Company 10 Pharmacovigilance Market Net Revenue, By Years, 2023-2030* ($)
Figure 160 Company 10 Pharmacovigilance Market Net Revenue Share, By Business segments, 2023 (%)
Figure 161 Company 10 Pharmacovigilance Market Net Sales Share, By Geography, 2023 (%)
Figure 162 Company 11 Pharmacovigilance Market Net Revenue, By Years, 2023-2030* ($)
Figure 163 Company 11 Pharmacovigilance Market Net Revenue Share, By Business segments, 2023 (%)
Figure 164 Company 11 Pharmacovigilance Market Net Sales Share, By Geography, 2023 (%)
Figure 165 Company 12 Pharmacovigilance Market Net Revenue, By Years, 2023-2030* ($)
Figure 166 Company 12 Pharmacovigilance Market Net Revenue Share, By Business segments, 2023 (%)
Figure 167 Company 12 Pharmacovigilance Market Net Sales Share, By Geography, 2023 (%)
Figure 168 Company 13 Pharmacovigilance Market Net Revenue, By Years, 2023-2030* ($)
Figure 169 Company 13 Pharmacovigilance Market Net Revenue Share, By Business segments, 2023 (%)
Figure 170 Company 13 Pharmacovigilance Market Net Sales Share, By Geography, 2023 (%)
Figure 171 Company 14 Pharmacovigilance Market Net Revenue, By Years, 2023-2030* ($)
Figure 172 Company 14 Pharmacovigilance Market Net Revenue Share, By Business segments, 2023 (%)
Figure 173 Company 14 Pharmacovigilance Market Net Sales Share, By Geography, 2023 (%)
Figure 174 Company 15 Pharmacovigilance Market Net Revenue, By Years, 2023-2030* ($)
Figure 175 Company 15 Pharmacovigilance Market Net Revenue Share, By Business segments, 2023 (%)
Figure 176 Company 15 Pharmacovigilance Market Net Sales Share, By Geography, 2023 (%)
 Email
Email Print
Print

